Consortium
The project involves:
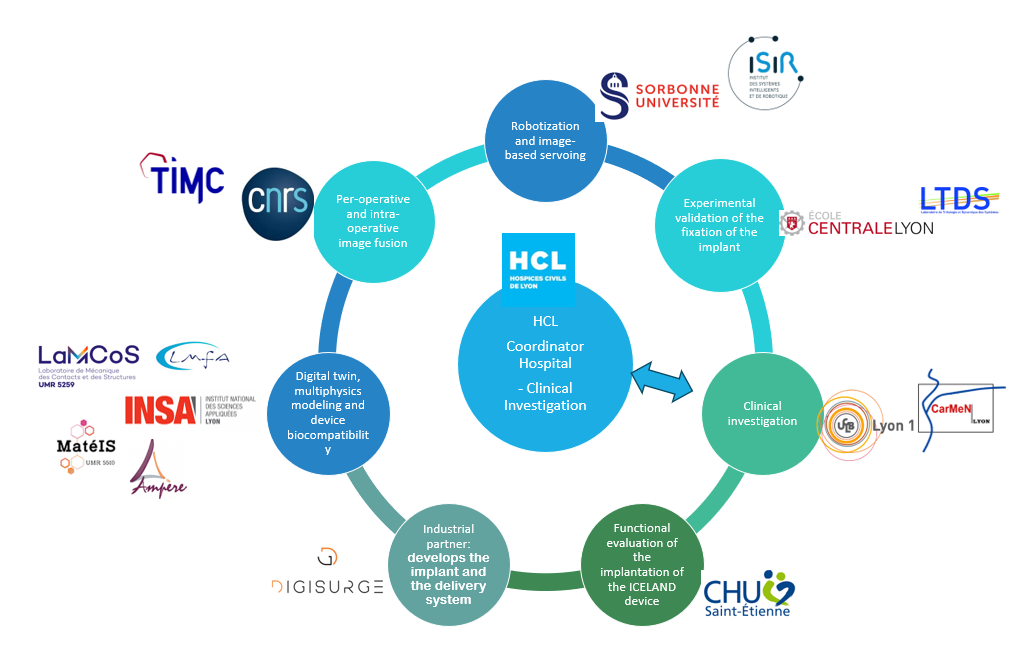
- Two University Hospitals:
- Hospices Civils de Lyon (HCL), the project coordinator institution, is in charge of the pre-clinical phase and of the clinical investigation aimed at producing evidence of efficacy and safety of the implant on the targeted mitral regurgitation population.
- CHU de Saint-Étienne (CHUSE) is in charge of the functional evaluation of the implantation of the ICELAND device for mitral valve repair.
- Five academic partners:
- Université Claude Bernard Lyon 1 (UCBL)
- Ecole Centrale de Lyon (ECL) is in charge of the experimental validation of the fixation of the implant on the mitral valve annulus
- INSA Lyon is in charge of several tasks :
- Numerical simulation of the treated valve
- Experimental validation of the anchoring solution
- Numerical simulation of the surgical procedure for surgeons training and surgery optimization
- Evaluation of the device’s biocompatibility
- Centre National de la Recherche Scientifique (CNRS) is in charge of pre-operative and intra-operative image fusion to :
- Assist with the surgery planning and the positioning of the surgical anchors
- Secure the intervention by using pre and per-operative information to motorize the robot.
- Sorbonne Université (SU) is in charge of the robotization of the delivery system and of guiding the robotic instrument owing to different imaging modalities
- A private company:
- DigiSurge develops the annuloplasty implant, the mechatronics catheter and delivery systems, the robotic platform integration, and will be in charge of market access and commercialization.
Consortium governance
The governance of ICELAND is composed of two entities:
- A Steering Committee composed by the project coordinator, the project manager and the stakeholders of the project.
- An independent Scientific Advisory Board in charge of scientific advising.
